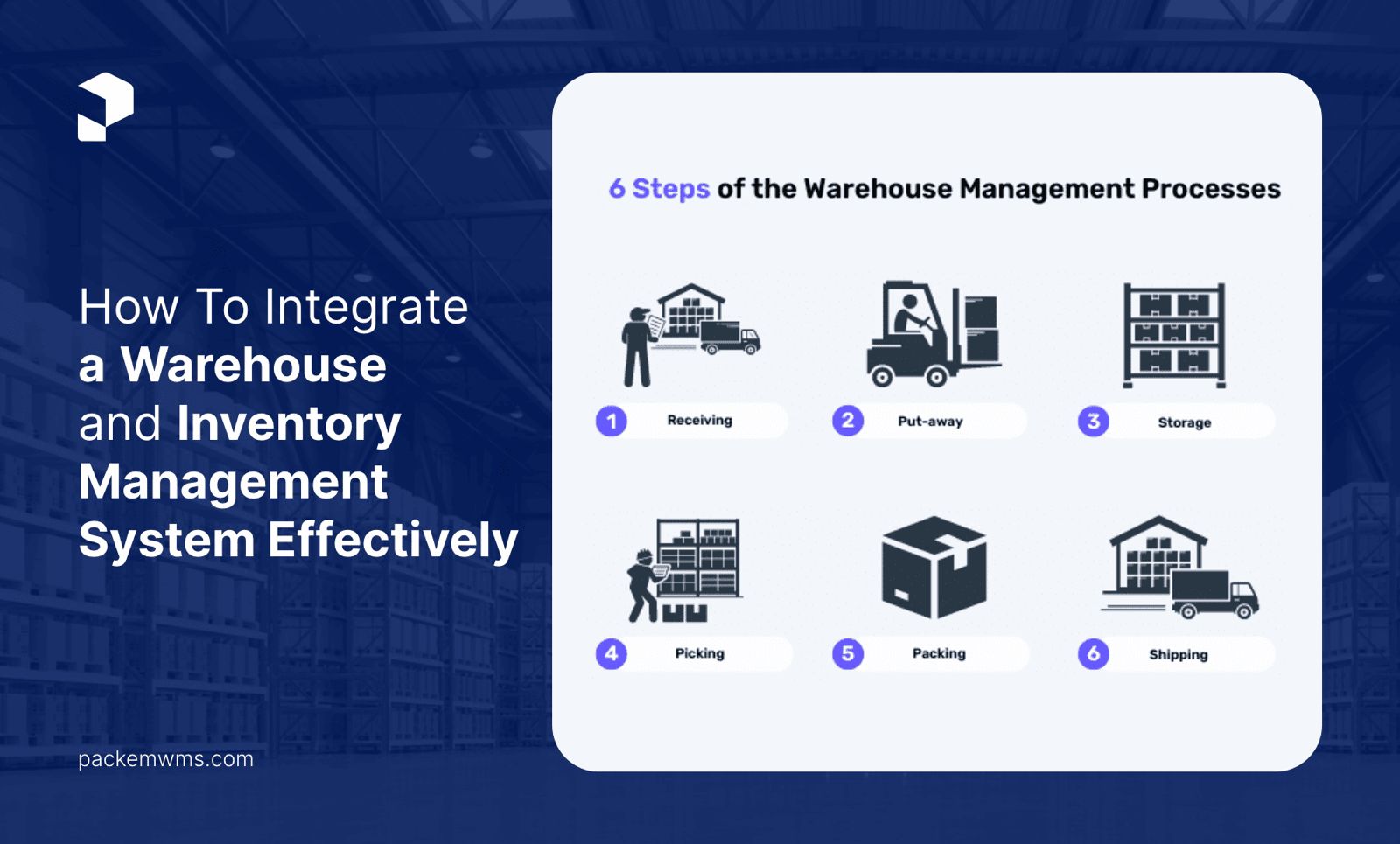
Built on the robust foundation of Amazon Web Services (AWS), Packem ensures the standards of data security and regulatory compliance.
This article delves into how PackemWMS aligns with the 21 CFR Part 11 requirements, demonstrating its commitment to data integrity and confidentiality.
FDA’s 21 CFR Part 11 Requirements And How Packem Stays Compliant:
1. Validation Of Systems
- PackemWMS undergoes validation processes, ensuring that it consistently meets predefined specifications and functionality requirements.
- Continuous monitoring and regular updates are conducted to maintain system integrity.
2. User Access Controls
- Robust user authentication mechanisms are in place, including secure login credentials, limiting access to authorized personnel.
- Passwords must be at least 8 characters in length, include a combination of uppercase and lowercase letters, numbers, and special characters, not contain any personally identifiable information, and be regularly changed.
- Access levels are defined, granting users appropriate permissions based on their roles within the system.
Experience the simplest inventory management software.
Are you ready to transform how your business does inventory?
3. Audit Trails
- Packem maintains comprehensive audit trails, capturing user activities, data changes, and system events.
- Timestamps, user identifications, and actions performed are logged, facilitating traceability and accountability.
4. Data Encryption
- All sensitive data within the WMS software is encrypted both in transit and at rest, using industry-standard encryption algorithms.
- Secure Socket Layer (SSL) and Transport Layer Security (TLS) protocols are employed to safeguard data during transmission.
5. System Security
- Packem is hosted on AWS, benefiting from the robust security measures provided by AWS, including firewalls, intrusion detection systems, and regular security audits.
- Regular vulnerability assessments and penetration testing are conducted to identify and mitigate potential security risks.
6. Data Archiving
- Packem includes a secure and compliant data archiving system, enabling organizations to retain and retrieve electronic records as required by 21 CFR Part 11 after signing the proper contracts.
- Archived data is stored in a tamper-evident manner to maintain data integrity.
Conclusion
In summary, PackemWMS serves as a HIPAA-compliant, multi-tenant warehouse management solution that goes above and beyond in meeting the stringent requirements of food and safety guidelines. By leveraging the power of AWS and implementing robust security measures, PackemWMS ensures data integrity, confidentiality, and regulatory compliance for healthcare organizations seeking a reliable and secure warehouse management solution.
If you are interested in chatting with our technical sales team, click the link Contact Us